Summary.
Human embryonic stem cells (HESCs) hold great promise for therapeutic intervention in a number of illnesses, especially in degenerative neurological conditions such as spinal cord injury, Huntington’s disease, Alzheimer’s disease, or degenerative retinal diseases. In principle, an HESC can differentiate to form any cell- or tissue-type in the body. Therefore, HESCs also provide a rare opportunity to create useful cell culture models of many human diseases, especially inherited diseases, in order to test hypotheses about molecular/cellular pathophysiology or potential therapies. Recent advances in creating so-called adult induced pluripotent stem cells (iPS cells) from skin biopsy samples of adult subjects also makes it possible to create iPS cell lines, or groups of cells lines, with a particular known genetic composition. Despite advances in stem cell biology, many fundamental basic questions remain to be addressed. For example, what is the basic molecular signature of a stem cell? What factors define “stemness” and what type of cellular environment maintains “stemness?” How can particular tissue or cell types be induced from stem cell cultures efficiently and reproducibly? At the heart of many of these questions is signal transduction, and at the heart of signal transduction is GPCR-mediated signaling. The Sakmar Laboratory has made a major commitment to investigating the role of GPCR-mediated signaling pathways, especially the familiar CXCR4-mediated signaling network, in stem cell biology.
The Role of G Protein Signaling Pathways is Stem Cell Differentiation.
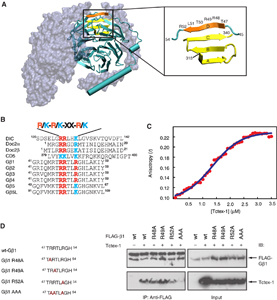
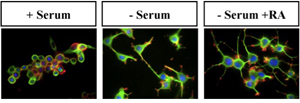
Recent work from the Sakmar Laboratory, in collaboration with Prof. Ching-Hwa Sung, Weill-Cornell Medical College, supports the idea that Tctex-1, a light-chain component protein of the dynein motor apparatus, and Gbg-dependent signaling play an important concerted role in neural stem cell fate determination. In ongoing work, Pallavi Sachdev of the Sakmar Laboratory is characterizing the functional consequences of the physical interaction between Tctex-1 and G proteins in neural stem cells and HESCs at both the cell and molecular biology levels. Dr. Sachdev collaborated to investigate the physiological relevance of the novel Gbg-Tctex-1 interaction in vivo using a state-of-the-art method called in utero electroporation to manipulate experimentally the neural stem cells in the developing rat neocortex. These studies clearly demonstrated that Tctex-1 suppression causes an overproduction of post-mitotic neurons at the expense of mitogenic neural stem cells.
Interestingly, Tctex-1 suppression induced phenotypes are strikingly similar to those caused by Gbg signaling disruption and suppression of other known G protein signaling activators. To further investigate the possible genetic interaction between Gbg and Tctex-1, Dr. Sachdev is testing the ability of Tctex-1 to rescue the phenotypes induced by Gbg signaling perturbation. These studies will be conducted both in vivo and in established human and mouse ES cells.
Searching for Drugs That Control Stem Cell Fate.
The logical next step in this work is to identify small molecules that might modulate the newly discovered Gbg-Tctex-1 complex and to evaluate their therapeutic potential. Sakmar Laboratory members have used their expertise with high-throughput screening (HTS) assays to develop an assay that employs the concept of homogeneous time-resolved fluorescence (HTRF), which combines the advantages of low background inherent in time-resolved fluorescence with an homogenous solution-based FRET approach. The screening strategy involves using His-tagged Tctex-1 and a biotin-labeled Gb-peptide coupled to Anti-His-cryptate and streptavidin XL-665 as the FRET donor and acceptor pairs. Using this assay a significant increase in FRET in the presence of the two interacting partners, Gb-peptide and Tctex-1 as compared with the peptide or protein alone controls was observed. Chemical compound libraries will be screened to identify small molecules that can be assayed later in mouse and human ES cell differentiation assays. Fundamental questions regarding the cellular and molecular mechanisms that control proliferation, differentiation and self-renewal of stem cells remain unanswered. Focusing on endogenous factors, including signal transduction pathways, that regulate stem cell fate will greatly accelerate and facilitate the therapeutic potential of ES cells.
|
|
 Membrane Biophysics | Computational Chemistry | Chemical Biology & Proteomics | Structural Biology | Nanoscience | Stem Cell Biology
Membrane Biophysics | Computational Chemistry | Chemical Biology & Proteomics | Structural Biology | Nanoscience | Stem Cell Biology
