TRANSMEMBRANE SIGNAL TRANSDUCTION BY G PROTEIN-COUPLED RECEPTORS
The Sakmar Laboratory uses genetic, biochemical and biophysical methods to study how signals from outside a cell are relayed across its membrane and into the cell interior, where they elicit responses via the process of signal transduction. Much of this work focuses on molecules known as G protein-coupled receptors (GPCRs), or seven-transmembrane heptahelical receptors, which play a key role in transducing a wide range of transmembrane signals.
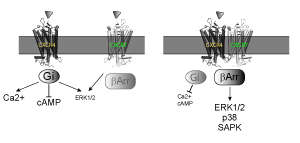
The mechanism of signaling by heptahelical receptors is an area of intense scientific interest and has tremendous biological and pharmaceutical relevance: heptahelical receptors represent the largest gene family in the human genome and the largest class of drug targets in the pharmaceutical industry. The Sakmar Laboratory is interested in understanding the precise structure of GPCRs in a membrane bilayer, the chemical principles underlying ligand recognition, and how receptors self-assemble to form higher-order structures and associate with membrane lipids and their cellular signaling partners. To accomplish these goals, the Sakmar laboratory employs an interdisciplinary approach involving cellular and molecular biology in combination with structural studies and advanced techniques of biophysics and computational chemistry.
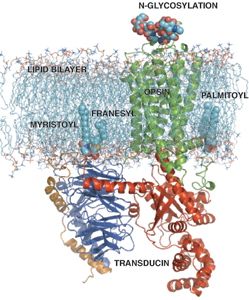
Dr. Sakmar and his colleagues have focused on visual pigments as a model system for biophysical studies and on chemokine receptors for studies of ligand recognition and proteomics. The Sakmar Laboratory has made significant contributions to vision research, including elucidating the physical basis of spectral tuning by visual pigments and the molecular mechanism underlying congenital “night blindness.” Studies of visual pigments helped the Sakmar Laboratory propose and test the “helix movement model” of receptor activation and the concept of “functional microdomains” in GPCRs.
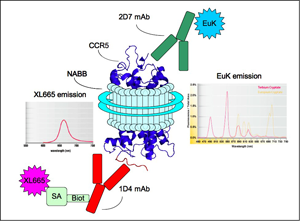
To study the molecular aspects of 7-TM receptor signaling in native-like membrane bilayers, the Sakmar Laboratory designed a novel, nanoscale, soluble, membrane-mimetic system coined the “NABB”, (Nanoscale Apolipoprotein Bound Bilayer) to use as a key element to elucidate the molecular mechanism of receptor signaling and oligomerization. The laboratory also employs advanced computational methods to obtain information about the behavior of GPCRs in membrane bilayers, using the simulations to guide experimental approaches that employ conformationally- or environmentally-sensitive fluorescent probes. Members of the Sakmar Laboratory built a fluorescence microscope workstation that will permit single-molecule detection (SMD) of labeled receptors in NABBS, or even live cells, and is also optimizing a method to label receptors in mammalian cells in vivo with unnatural amino acids. Adaptation of an amber codon suppression technology to carry out site-directed unnatural amino acid mutagenesis in GPCRs expressed in mammalian cells in culture represents a major recent technical advance.
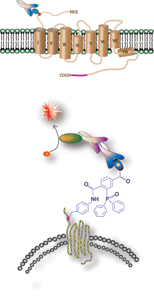
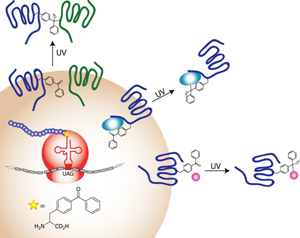
To understand receptor-ligand recognition, the Sakmar Laboratory focuses on heptahelical chemokine receptors, which control cell migration and also act as co-receptors for HIV-1 cellular entry. Dr. Sakmar and his colleagues are developing methods to prepare and analyze peptides with defined patterns of a post-translational modification, tyrosine sulfation. They have also developed a method that allows self-assembly of an immobilized membrane bilayer containing oriented recombinant expressed chemokine receptors. This membrane protein “chip” device is amenable to single-molecule detection using total internal reflectance fluorescence (TIRF) microscopy and microfluidics.
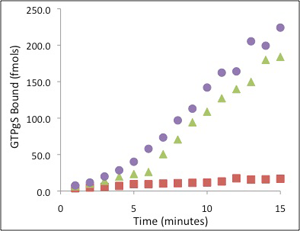
Finally, to understand how multiple cellular signaling pathways communicate, the Sakmar Laboratory has made a major drive to identify novel binding partners and regulators of cellular G proteins, and to obtain structures of important protein-protein complexes. The downstream cytoplasmic components of G protein signaling pathways regulate signal intensity and turn-off. Members of the Sakmar Laboratory have a particular interest in defining non-classical protein-protein interactions that modulate cross talk between signaling pathways – work that has relevance to cancer biology and stem cell biology.
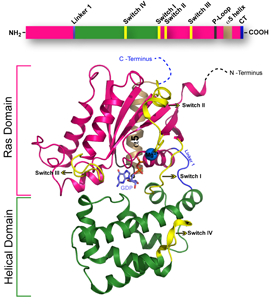
For example, the Sakmar Laboratory discovered that the b subunit of heterotrimeric G proteins interacts with a dynein light-chain component called Tctex-1 to control neurite outgrowth in developing neurons and to regulate differentiation of embryonic stem cells to neuronal lineages. They also discovered that the adapter/kinase regulator protein b-PIX interacts with the E3-ubiquitin ligase AIP4 to modulate CXCR4 chemokine receptor signaling.
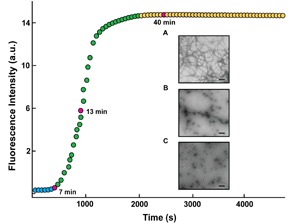
Studies of the regulation of downstream signaling also lead to the serendipitous discovery of a calcium-binding protein, called NUCB1, which binds to amyoidogenic peptides, including amylin and Ab42, and prevents their assembly into amyloid fibrils. This discovery has major implications for understanding the precise mechanism of amyloid fibril formation, which is associated with approximately 20 human diseases, and how to prevent amyloid formation in patients.
|
 Membrane Biophysics | Computational Chemistry | Chemical Biology & Proteomics | Structural Biology | Nanoscience | Stem Cell Biology
Membrane Biophysics | Computational Chemistry | Chemical Biology & Proteomics | Structural Biology | Nanoscience | Stem Cell Biology