Summary.
Structural biology refers the general field of research where high-resolution structures of macromolecules are determined. The ultimate goal of structural biology is to correlate structure with biological function. The two primary methods used to determine high-resolution structures of proteins and nucleic acids are X-ray crystallography and NMR (nuclear magnetic resonance) spectroscopy. Although the vast majority of high-resolutions structures have been obtained using X-ray crystallography, these two methods are generally considered to be complementary. Over the past 5 years, the Sakmar Laboratory has developed an active research program in structural biology using both X-ray crystallography and NMR spectroscopy to study proteins involved in GPCR signaling pathways. Members of the Sakmar Laboratory also employ structures reported by others, especially X-ray crystal structures of GPCRs, to facilitate molecular dynamics computer simulations. The major structural biology projects currently active in the Sakmar Laboratory are described below. NMR Structural Constraints Define the Active State of Rhodopsin.
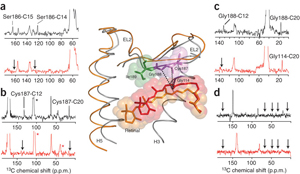
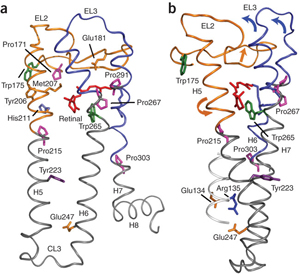
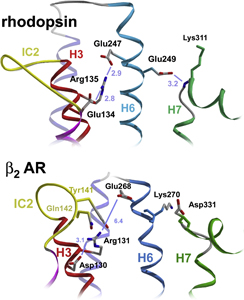
The second extracellular loop of rhodopsin forms a cap over the binding site of its photoreactive 11-cis retinal chromophore. A critical question has been whether EL2 forms a reversible gate that opens upon activation or whether it acts as a rigid barrier. In collaboration with Prof. Steven O. Smith, Stony Brook University, the Sakmar Laboratory used magic angle spinning (MAS) solid-state 13C NMR spectroscopy to measure distances between the retinal chromophore and the b4 strand of EL2 in the dark and after light activation. The results confirmed that the EL2 loop moves away from the retinal binding site upon activation, and that there is a rearrangement in hydrogen-bonding networks connecting EL2 with the extracellular ends of transmembrane helices. The structural changes in EL2 are coupled to the motion of helix 5 and breaking of an ionic lock structure that regulates activation. These results provide a comprehensive view of how retinal isomerization triggers helix motion and activation in rhodopsin and serve as a model for how other GPCRs might also work.
NMR Structure of an Engineered Form of Chemokine CXCL12.
The chemokine CXCL12 (SDF1) and its GPCR binding partner CXCR4 orchestrate cell migration, which occurs during inflammation, stem cell homing and breast cancer metastasis. In addition, over 20 cancer types express CXCR4 and metastasize to tissues that secrete SDF1, including bone marrow, lung, liver and lymph nodes. In order to elucidate the structural basis of the binding of SDF1 to CXCR4, the Sakmar Laboratory collaborated with Prof. Brian F. Volkman, Medical College of Wisconsin, to determine the solution NMR structure of an engineered constitutively dimeric SDF1 in complex with a CXCR4 N-terminal peptide fragment. The 38-amino acid residue N-terminal peptide contained three sulfotyrosine residues that were determined to be important for high affinity ligand-receptor interaction. The structure shows that a single CXCR4 peptide bridges the SDF1 dimer interface. Sulfotyrosines sY7 and sY12 occupy positively charged clefts on opposing chemokine subunits.
Functional analysis of SDF1 mutants suggested that a salt bridge between sY21 of CXCR4 and R47 of SDF1 also contributes to binding affinity. The engineered dimeric SDF1 induced intracellular Ca2+-flux, but surprisingly had no effect on chemotactic activity. Instead, the engineered dimer prevents native SDF1-induced chemotaxis, suggesting that it has features of a potent partial agonist. This work elucidated the structural basis for sulfotyrosine recognition in chemokine-receptor interaction and suggested a novel strategy for CXCR4-targeted drug development, which is being pursued in follow-up work in the Sakmar Laboratory.
Structures of Mutant G Proteins Suggest a Sequential Release Activation Mechanism.
Heptahelical GPCRs couple to heterotrimeric G proteins to relay extracellular signals to intracellular signaling networks, but the molecular mechanism underlying GDP release by the G protein α-subunit is not well understood. Amino acid substitutions in the conserved α5 helix of Gi, which extends from the C-terminal region to the nucleotide-binding pocket, cause dramatic increases in basal (receptor-independent) GDP release rates. For example, mutant Gαi1-T329A shows an 18-fold increase in basal GDP release rate. The Sakmar Laboratory determined the crystal structure of mutant Gαi1-T329A•GDP, which showed substantial conformational rearrangement of the switch 1 region and additional striking alterations of side chains lining the catalytic pocket that disrupt the Mg+2 coordination sphere and dislodge bound Mg+2. Based on the model the Sakmar Laboratory proposed a “sequential release” mechanism whereby a transient conformational change in the α5 helix alters switch 1 to induce GDP release. Interestingly, this mechanistic model for heterotrimeric G protein activation is similar to that suggested for the activation of the plant small G protein Rop4 by RopGEF8.
Crystal Structures of Proteins That Regulate Downstream Kinase Signaling Pathways.
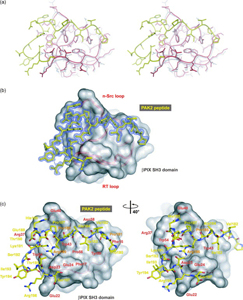
The Sakmar Laboratory is interested in the molecular details governing protein-protein interactions in non-canonical GPCR signal transduction pathways – pathways that are primarily mediated by proteins other than heterotrimeric G proteins. In ongoing structural studies, the interactions of bPIX (p21-interacting exchange factor) with both PAK (p21-activated kinase) and Cbl (Casitas B cell lymphoma) are being elucidated. Members of the Sakmar Laboratory are determining how bPIX-PAK and Cbl-PAK complexes are regulated by Gbg subunits generated from ligand activated CXCR4 receptors.
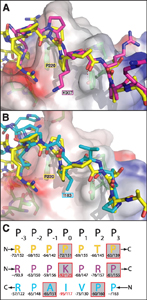
Reversible protein-protein interactions, which play crucial roles in cellular signal transduction pathways, are often mediated through recognition domain modules. bPIX-PAK complex formation is mediated by the SH3 (Src-homology 3) domain of bPIX, but unlike typical SH3 domain interactions, the recognition occurs, through an unconventional non-PxxP (PRP) peptide sequence. How does the unusual PAK peptide sequence recognize and bind the bPIX SH3 domain? To address this question, the Sakmar Laboratory solved the crystal structure of the SH3 domain of bPIX in complex with a peptide corresponding to the PAK1 bBIX binding site at 1.30 Å resolution. Interestingly, the asymmetric unit of the crystal structure reveals that the bPIX SH3 domain dimerizes upon binding the PAK1 peptide, making peptide contacts at the dimer interface. Importantly, this dimer shares striking similarity with the “super-SH3” domain of p47phox from NADPH oxidase which forms an intermolecular dimer that is regulated by phosphorylation.
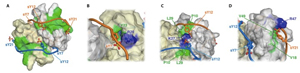
Cross-talk between GPCR and RTK (receptor tyrosine kinase) signaling pathways are crucial to the efficient relay and integration of cellular information. The Sakmar Laboratory identified a novel binding interaction of the E3 ubiquitin ligase AIP4 with the GTP exchange factor bPIX. The crystal structure of the bPIX–SH3/AIP4 complex was determined at 2.0 Å resolution and showed that AIP4 binds the bPIX–SH3 domain as a canonical Class I ligand with an additional type II polyproline helix that makes extensive contacts with another face of bPIX. Taken together, the novel interaction between AIP4 and bPIX represents a new regulatory node for GPCR and RTK signal integration. The structure of the bPIX–SH3/AIP4 complex provides important insight into the mechanistic basis for bPIX scaffolding of signaling components, especially those involved in cross-talk.
|
|
 Membrane Biophysics | Computational Chemistry | Chemical Biology & Proteomics | Structural Biology | Nanoscience | Stem Cell Biology
Membrane Biophysics | Computational Chemistry | Chemical Biology & Proteomics | Structural Biology | Nanoscience | Stem Cell Biology




