| |
Summary.
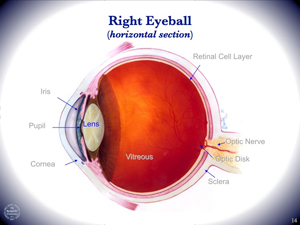
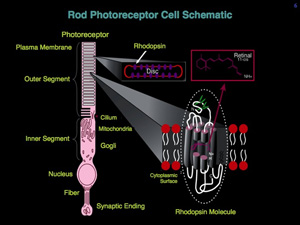
Visual phototransduction is the process whereby a photon of light is captured by the eye and converted into a chemical signal that can be relayed to the brain and perceived. In studies of visual perception, the Sakmar Laboratory uses the proteins rhodopsin and transducin as models for structure-function studies on the molecular mechanism of transmembrane signaling. These visual proteins, which are found in the retina, are members of a super-family of related GPCRs (G protein-coupled receptors) and heterotrimeric G proteins (guanine nucleotide-binding regulatory proteins), respectively. Light-activated rhodopsin catalyzes guanine-nucleotide exchange by transducin, which ultimately leads to a change in membrane ion conductance and a neural signal. In particular, rhodopsin, the dim-light photoreceptor in the rod cell in the retina, is an excellent prototype for biophysical studies of the GPCR superfamily.
Approaches to Studies of Rhodopsin Activation Mechanism.
One general approach used by the Sakmar Laboratory is to clone and characterize genes for relevant signaling pathways and then reconstitute expressed proteins in defined in vitro systems. Biochemical and biophysical methods are then used to examine the functional significance of specific protein domains or amino acid residues. The Sakmar Laboratory has studied the ground-state structure of rhodopsin, the interactions between specific amino acid residues and its vitamin A chromophore that control spectral properties and photochemistry, the mechanism by which a photochemical signal is transmitted from the core of the receptor to its cellular surface, and the specific domains on the cellular surface that bind and activate transducin.
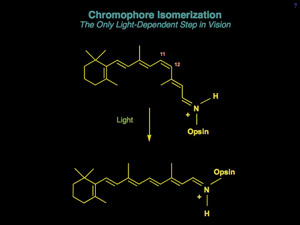
Rhodopsin undergoes a well-documented series of conformational changes initiated by the absorption of a photon of visible light. Molecular biological approaches in combination with various spectroscopic and biophysical methods have allowed the study of recombinant mutant pigments to address the molecular mechanism of receptor activation. It has been known since the 1960’s that rhodopsin contains an 11-cis retinylidene (vitamin A) chromophore, which isomerizes to the all-trans conformation upon photon absorption. But, how is chromophore isomerization coupled to receptor activation? How do the conformational changes in the chromophore-binding pocket of rhodopsin propagate to the intracellular surface of the receptor? What are the potential electrostatic changes associated with these conformational changes? The dominant interaction between chromophore and protein is an electrostatic interaction involving the positively-charged chromophore and its protein counterion, a glutamic acid residue. However, it is likely that both steric and electrostatic factors contribute to receptor activation. At least five concerted events are known to occur in forming the active receptor conformation: 1) retinal isomerization, 2) charge neutralization of the retinal, 3) protonation of the counterion, 4) movement of transmembrane segments, primarily helical segments 3 and 6, and 5) proton uptake at the cellular surface of the receptor. Charge neutralization and subsequent protein conformational changes are likely to be driven by electrostatic interactions.
The mechanistic details of electrically-active conformational changes in membrane proteins are of great interest. Since it is not unusual for biological membranes to have significant transmembrane potentials, membrane proteins may be exposed to intense electric fields. Their structural conformations may be functionally coupled to the electric field for the purpose of switching, transport, or energy transduction. In rhodopsin a flash of light can change the conformational state, and the conformational changes can be probed by a variety of spectroscopic techniques including time-resolved absorption spectroscopy. Exciting new methods have been applied to study the relationship between structure and electrostatics in membrane protein receptors. It is reasonable to expect that these methods will bring a new dimension to structure-function studies of rhodopsin and other visual pigments.
Tuning Color Visual Pigments.
Essentially all animals – from human to birds to fishes to insects – contain visual pigments with the same light-absorbing molecule, a derivative of vitamin A. However, they can all detect wavelength-dependent light – that is, they can see colors, in many cases from near ultraviolet (UV) to deep red. Humans, for example, use rhodopsin in rod cells for dim-light vision, and they use red, green and blue pigments in cone cells for bright light high-acuity trichromatic color vision. But all of these visual pigments use the same vitamin A chromophore, called 11-cis-retinal, which is the ligand for the protein opsin.
Human trichromatic color vision depends on the presence of three distinct types of cone cells in the retina. Mutations in the genes for human visual pigments cause a variety of congenital color blindness syndromes. Molecular genetic analysis of mutant genes provided the first clues about how visual pigments tune to absorb specific wavelengths of light. Each cone – blue, green, and red – contains a different pigment. However all of the pigments use the same chromophore. What is the physical mechanism underlying spectral tuning? How do amino acid residues interact with the chromophore to tune to wavelengths of light from ultraviolet to infrared?
The Sakmar Laboratory addressed these questions in collaboration with Prof. Richard A. Mathies, University of California at Berkeley, and developed a method to record resonance Raman spectral of recombinant expressed visual pigments. Raman spectroscopy provides detailed and specific information about the chemical bond vibrations of the chromophore. They determined that the predominant chemical mechanism in spectral tuning involves interactions of dipolar amino acid residues with the ground-state and first excited-state molecular orbital charge distributions of the chromophore. Mutation or evolution of visual pigment genes can alter these interactions to cause spectral shifts that affect color perception. Understanding the physical chemistry of spectral tuning has provided a complete understanding of the molecular genetics of color blindness.
Phototransduction Mechanism.
Sakmar Laboratory members also determined which amino acids in rhodopsin were responsible to transmit the signal of light detection into a biochemical signal. This process is known as phototransduction. Members of the Sakmar Laboratory developed new methods to monitor rhodopsin’s light-dependent interaction with its G protein, called transducin. They used these methods to study which amino acids of rhodopsin were required for signal transduction. Some amino acids were required to elicit specific conformational changes in rhodopsin, while others were required to facilitate interaction with the G protein. Ultimately, a light-dependent change in the shape of 11-cis-retinal in the core of the receptor causes transducin to swap one nucleotide, GDP, for another, GTP. This overall process is particularly interesting because the signaling machinery has to sense the change in shape of the bound ligand and transmit the signal to the GDP-binding pocket of transducin – a distance of approximately 7 to 8 nm!
In the course of this work, which involved significant interdisciplinary collaborations with the laboratories of Prof. Henry R. Bourne, Prof. Steven O. Smith, Prof. David S. Kliger, Prof. Friedrich Siebert and Prof. Klaus Peter Hofmann, the Sakmar Laboratory proposed and established several important concepts in the field, such as: “Functional Micro Domains” in GPCRs, the “Helix Movement Model of Receptor Activation,” and the “Sequential Release Model of Nucleotide Exchange.”
Ten years of site-directed mutagenesis work was largely validated when the high-resolution crystal structure of bovine rhodopsin was published in 2000. Using the structure to design rational experiments, members of the Sakmar Laboratory continued to pioneer the use of new biophysical approaches. For example, Dr. Elsa Yan, currently at Yale University, used density functional theory calculations and resonance Raman spectroscopy to propose the “Counterion Switch Mechanism” of rhodopsin activation and to describe energy storage in the early photoproducts of rhodopsin. High-level expression methods also allowed Dr. Yan to collaborate with Prof. Steven O. Smith to obtain informative solid-state NMR (nuclear magnetic resonance) structures of rhodopsin mutants with isotopic labels at specific amino acids or at specific locations in the retinal ligand – structures that show the sequence of coupled movements in the receptor protein after the retinal is activated by light.
Visual Disorders.
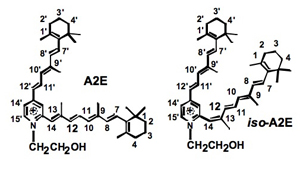
How the vitamin A ligand is recycled in the retina is relevant to understanding the molecular pathophysiology of several visual disorders, including autosomal dominant retinitis pigmentosa (ADRP) and age-related macular degeneration (AMD). The Sakmar Laboratory has studied how the accumulation of vitamin A in the retina might lead to the formation of a toxic byproduct called, A2E. A key factor in preserving vision with aging is to protect the health of photoreceptor cell membranes in a dynamic oxygen rich environment that might be prone to damage by oxidants and free radicals. How does rhodopsin function in the cell membrane and how does vitamin A move in and out of its binding site. Dr. Thomas Huber has generated useful MD (molecular dynamics) computer models of rhodopsin and other class A receptors in model membranes to develop a new theory of how GPCR ligands enter their binding pockets. He also employed coarse-grained MD simulations of multiple rhodopsin receptors in a model membrane bilayer to study receptor oligomerization in collaboration with Dr. Xavier Periole and Prof. Seiwert-Jan Marrink of University of Groningen, The Netherlands.
Sensory Neuroscience Meets Systems Biology.
Visual phototransduction is directly related to other forms of cell-cell communication and to other sensory systems, such as olfaction (sense of smell) and taste in higher organisms, and chemotaxis in lower organisms. Chemotaxis refers specifically to the chemical detection system that is coupled to directed movement. For example, an organism responds to a toxic chemical repellant by moving away from the stimulus, and to a chemical attractant by moving toward the stimulus. Chemosensory receptors in organisms as simple as yeast cells are heptahelical receptors. Insects also employ heptahelicals to detect volatile chemical signals.
The Sakmar Laboratory recently collaborated with the laboratory of Prof. Leslie B. Vosshall at Rockfeller University to develop a method to create and measure precise gradients of volatile odorants in space. They adapted a method pioneered earlier by the Sakmar Laboratory called Fourier-transform infrared (FTIR) spectroscopy and invented a sample chamber in which the movements of fruit fly (Drosophila) larvae can be tracked simultaneously while measuring odorant gradients using the FTIR device. The method provides a digital record of larvae movements under various conditions. The combination of the new tracking device and fly genetics provided insights into understanding the precise sensitivity and spatial resolution of fly chemotaxis.
|
|
 Drug Development | Vision & Sensory Neuroscience | AIDS | Amyloid Disease
Drug Development | Vision & Sensory Neuroscience | AIDS | Amyloid Disease