 |
EXAMINING RECEPTOR ACTIVATION
Tagging GPCRs with azido probes
Heptahelical G protein-coupled receptors (GPCRs) in cell membranes act as molecular switches. Precisely how
receptors turn on in response to ligand binding is not known. To track the earliest protein conformational changes in the
receptor activation pathway, Shixin Ye of the Sakmar Lab and an international team of collaborators engineered
rhodopsin, a prototypical GPCR, to contain unique azido probes. The engineered receptors were interrogated by FTIRdifference
spectroscopy to show that certain light-induced conformation changes happen much more quickly than had
been expected.
The work was published online in Nature on April 11, 2010.
Tracking G-protein-coupled receptor activation using genetically encoded infrared probes
doi:10.1038/nature08948
[pubmed online abstract]
J Mol Biol. 2009 Aug 21. [Epub ahead of print]
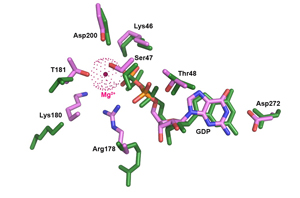
Structural Evidence for a Sequential Release Mechanism for Activation of Heterotrimeric G Proteins.
Kapoor N, Menon ST, Chauhan R, Sachdev P, Sakmar TP.
Laboratory of Biochemistry and Molecular Biology, Rockefeller University, 1230 York Avenue, New York, NY 10065, USA.
Heptahelical G protein-coupled receptors (GPCRs) couple to heterotrimeric G proteins to relay extracellular signals to intracellular signaling networks, but the molecular mechanism underlying GDP release by the G protein alpha-subunit is not well understood. Amino acid substitutions in the conserved alpha5 helix of G(i), which extends from the C-terminal region to the nucleotide-binding pocket, cause dramatic increases in basal (receptor-independent) GDP release rates. For example, mutant Galpha(i1)-T329A shows an 18-fold increase in basal GDP release rate, and when expressed in culture it causes a significant decrease in forskolin-stimulated cAMP accumulation. The crystal structure of Galpha(i1)-T329A*GDP shows substantial conformational rearrangement of the switch I region and additional striking alterations of side chains lining the catalytic pocket that disrupt the Mg(+2) coordination sphere and dislodge bound Mg(+2). We propose a "sequential release" mechanism whereby a transient conformational change in the alpha5 helix alters switch I to induce GDP release. Interestingly, this mechanistic model for heterotrimeric G protein activation is similar to that suggested for the activation of the plant small G protein Rop4 by RopGEF8.
[PMID: 19703466 [PubMed - as supplied by publisher]
|
|







