|
Summary.
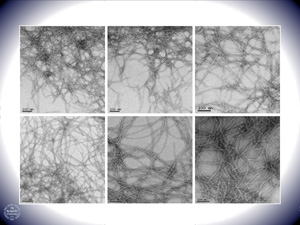
The aggregation of hydrophobic peptides into amyloid fibrils is a characteristic pathological feature of a number of diseases, including Type 2 diabetes, Alzheimer’s disease, Parkinson’s disease and Huntington’s disease. Amyloid fibrils and their pre-fibrillar aggregates exhibit toxicity in cell culture based assays and it is generally believed that to prevent aggregation of pathogenic amyloid peptides would prevent diseases. Members of the Sakmar Laboratory recently discovered a unique property of a human Ca2+-binding protein, Calnuc, or nucleobindin 1 (NucB1). NucB1 can inhibit the aggregation, at both nucleation and pre-fibrillar stages, and decrease the neurotoxicity in cell viability assays of amylin and Aβ42. Amylin and Aβ42 are amyloidogenic peptides implicated in the pathophysiology of adult-onset diabetes mellitus and Alzheimer’s disease, respectively. To understand the mechanism of action of this important property of NucB1, and to exploit its potential as a possible therapeutic agent, is a major ongoing focus of the Sakmar Laboratory.
Common Features of Amyloid Disease.
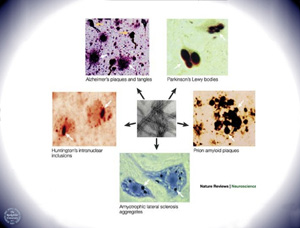
More than thirty diseases, including several neurodegenerative disorders like Alzheimer’s and Parkinson’s disease, are characterized by the formation of extracellular amyloid fibrils. These filamentous deposits ranging in size from a few nanometers to microns are protein aggregates with parallel or anti-parallel alignments of β-strands in β-sheets. Interestingly, each of these diseases involves a natively unfolded or a misfolded protein that shares no inherent sequence or structural similarity with the other amyloidogenic peptides. Since the discovery of proteinaceous plaques in the demented patients by Alois Alzheimer in 1901, several research groups have actively investigated the cause of amyloid formation and pathways leading to its neurotoxicity. A major focus of research for the past decade has aimed at preventing the formation of CNS amyloid, or devising strategies to remove amyloid or reduce its toxicity once it has formed.
Molecular Pathophysiology of Alzheimer’s Disease. Amyloid fibrils associated with Alzheimer’s disease are composed of a naturally-occurring amyloidogenic peptide called Aβ42. It was observed that exposure of cultured neurons to synthetic Aβ solutions containing fibrils leads to cell death. However, the size and number of fibrillar deposits did not correlate with the severity of the disease. As an alternative, Teplow and others postulated that pre-fibrillar aggregates like protofibrils, Aβ-derived diffusible deposits or Aβ oligomers might be the cause of toxicity. It was further detected that under normal physiological conditions the brain produces small quantities of Aβ.
Initial research involving Alzheimer’s disease aimed at understanding aggregation and finding small molecule inhibitors against amyloidogenesis that could be tested as potential drug leads. In the past decade, however, the focus of understanding Alzheimer’s has moved from small molecule inhibitors to discovering proteins that keep these naturally translated hydrophobic peptides in solution. Indeed, a significant number of heat shock proteins (Hsp’s) and small heat shock proteins (sHsp’s) have been unveiled that inhibit aggregation of amyloidogenic peptides. Hsp104 inhibits the fibrillization of Sup35 prion conformers. Several members of sHsp’s like αB-crystallin, Hsp27, Hsp20 and HspB8 bind to Aβ40 and Aβ42 and completely inhibit the aggregation of Aβ40 into mature fibrils. Incubation of Aβ40 with these sHsp’s completely abolished cerebrovascular toxicity. The extracellular chaperone Clusterin has also been shown to inhibit aggregation of prion protein, Aβ and apolipoprotein CII.
Both Hsp70 and Clusterin are believed to bind to prefibrillar species and prevent the formation of β-sheet amyloid. The stabilization of these prefibrillar species, however, proved to be toxic in a cell culture based assay. Another mechanism to decrease Aβ load in brain is the proteolytic activity of Insulin Degrading Enzyme (IDE) and Neprilysin (NEP). IDE is a cytosolic protein that has been shown to degrade Aβ monomers. IDE hypo-function produces accumulation of β-amyloid peptide and induces glucose intolerance. NEP is a plasma membrane protein with its catalytic site exposed extracellularly to target Aβ diffuse deposits and neuritic plaques. The activity of these enzymes together regulates the amount of Aβ species present in the brain. A third pathway of Aβ clearance operates through Low-density lipoprotein receptor-related protein (LRP). LRP is believed either to bind directly to Aβ or to its chaperone complex with LRP ligands α2Microglobulin or apolipoprotein E4. The binding to LRP triggers internalization of the complex to late endosomes after which they are either delivered to lysosomes for degradation or effluxed across the blood brain barrier.
Molecular Pathophysiology of Diabetes.
Amylin (hIAPP, human islet amyloid polypeptide) is a 37-amino acid residue peptide co-secreted with insulin by pancreatic beta cells. The abnormal deposition of amylin and its formation into toxic fibrils in the pancreas and other tissues has been implicated as an etiology in certain cases of adult-onset diabetes mellitus. As with many other amyloid diseases, to prevent the formation or amylin-derived fibrils, or to remove the fibrils once they have formed, might be beneficial in treating diabetes.
Recent Work on NucB1. The recent discovery by members of the Sakmar Laboratory that NucB1 can prevent fibril formation by Aβ42 peptides is remarkable. The potential use of NucB1, or an engineered form of NucB1, as a therapeutic agent for amyloid disease, including Alzheimer’s, is an extremely exciting possible long-term result of ongoing work.
|
|
 Drug Development | Vision & Sensory Neuroscience | AIDS | Amyloid Disease
Drug Development | Vision & Sensory Neuroscience | AIDS | Amyloid Disease