Summary.
The Sakmar Laboratory has taken a leading role applying principles of computational chemistry to the study of GPRC structure and the molecular mechanism of GPCR-mediated transmembrane signaling. As described below, the hallmark of the interdisciplinary approach in the Sakmar Laboratory has been to carry out computational studies in parallel with complementary experimental methods.
Pioneering Computer Models of GPCRs.
Early computer models of the transmembrane region of rhodopsin were based on low-resolution cryo-electron microscopy images of rhodopsin in 2-dimensional arrays. These early models proved useful to create homology models of chemokine receptor CCR5, which were used in combination with site-directed mutagenesis to elucidate the binding sites of small molecule HIV-1 entry blockers. These studies evolved over time as computational modeling methods improved. Chemokine receptor models are now widely used in conjunction with medicinal chemistry to optimize structures of drug candidates.
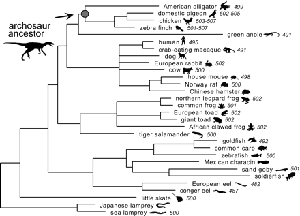
The Sakmar Laboratory also pioneered the development of ancestral gene reconstruction methods. Based on detailed phylogenetic analysis of opsin genes, the Sakmar Laboratory deduced the amino acid sequences of extinct archosaur opsins, synthesized the corresponding genes, expressed the genes in culture, and showed that the expressed archosaur proteins could function to absorb light and transmit signals.
GPCR Structural Dynamics in Membranes.
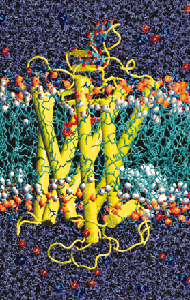
Beginning in 2000 with the publication of the high-resolution crystal structure of rhodopsin, members of the Sakmar Laboratory also created some of the first all-atom molecular dynamics (MD) models of rhodopsin in a model bilayer. All-atom MD models can be created for many GPCRs now that more crystal structures are available and computing power and force-field algorithms have improved. For example, the Sakmar Laboratory recently reported a long time-scale (200 ns) MD simulation of the b2-adrenergic receptor in a bilayer. However, a complete rendering of an individual GPCR in a bilayer might require 70,000 or more individual atoms, which is at the limit of current technology. To address the important issue of how GPCRs assemble in bilayer, the Sakmar Laboratory has pioneered the application of coarse-grain MD simulations, where groups of atoms are approximated by single bits of data. CG-MD can be used to model the behavior of collections of GPCRs in model bilayers.
High performance MD simulations can also be employed to study, especially in the context of complementary experimental data, the properties of the GPCR “signalosome,” which can be defined as a complex of proteins that assemble at the membrane to transmit signals. One example of a GPCR “signalosome” is the complex comprised of an active receptor bound to a heterotrimeric G protein.
Computational Approaches to Elucidating Rhodopsin Function.
The visual pigment rhodopsin is a prototypical GPCR, but is also has unique and interesting photochemical properties that can be addressed using computational chemistry. For example, the Sakmar Laboratory, in collaboration with Prof. Richard A. Mathies, used resonance Raman spectroscopy and ab initio density theory calculations to investigate the origin of energy storage in early rhodopsin photoproducts. They concluded that a unique twisted geometry of the rhodopsin chromophore immediately after isomerization traps energy and accounts for a peculiar hydrogen-out-of-plane (HOOP) chromophore vibrational frequency.
How the retinal chromophore exits its binding pocket after photobleaching, and how a new molecule of retinal enters to restore visual photosensitivity has also been addressed using interdisciplinary approaches. Starting with a detailed MD model of rhodopsin in the bilayer, and using a collection of homology models of visual pigments with different rates of retinal uptake, the Sakmar Laboratory predicted specific sites for retinal entry in the transmembrane regions of rhodopsin and other receptors. These results have profound implications for understanding the physiology of visual dark adaptation and for predicting how hydrophobic ligands, which partition into the membrane bilayer from the aqueous phase, enter the active sites of GPCRs.
|
|
 Membrane Biophysics | Computational Chemistry | Chemical Biology & Proteomics | Structural Biology | Nanoscience | Stem Cell Biology
Membrane Biophysics | Computational Chemistry | Chemical Biology & Proteomics | Structural Biology | Nanoscience | Stem Cell Biology
