 |
 Shixin Ye, Postdoctoral Associate Shixin Ye, Postdoctoral Associate
Laboratory of Molecular Biology and Biochemistry
Rockefeller Research Building 510
The Rockefeller University, Box 187
1230 York Avenue
New York, NY 10065
Telephone: 212-327-8284
Fax: 212-327-7904
sye@rockefeller.edu
|
|
Education:
Postdoctoral Researcher, Rockefeller University 2004 - present
Ph.D. in Chemistry, University of Pennsylvania 1998 - 2004
B.S. in Chemistry, Peking University 1994 - 1998
|
|
 Research: Research:
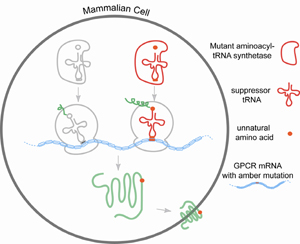
G protein-coupled receptors (GPCRs) belong to a seven transmembrane family of membrane proteins that are involved in diverse signaling processes. Current knowledge about this family of membrane proteins is largely hampered by the difficulties in sample preparation and the lack of varieties of structural sensitive techniques. My major focus is to probe GPCRs by site-specifically incorporating unnatural amino acids through the amber condon suppression technology. Amber codon suppression technology involving readthrough of an amber codon (UAG) in an mRNA by a suppressor tRNA aminoacylated with the unnatural amino acid has been an important recent advance in molecular biology (Noren et al., Science, 1989). Establishment of this methodology has provided the flexibility to modify protein with chemical groups not present in any of the 20 amino acids. Various amino acid analogues with novel chemical, physical, and biological properties have been incorporated into model proteins (Wang et al., Chemistry and Biology, 2009). Recently, we have optimized an expression system in mammalian HEK293T cells to incorporate p-acetyl-L-phenylalanine (a chemical handle), p-benzoyl-L-phenylalanine (a chemical handle and a photo-crosslinker) and p-azido-L-phenylalanine (a chemical handle, an IR probe, and photo-crosslinker) efficiently into GPCRs (Ye et al., JBC, 2008)
I am currently working on two research projects, with the application of unnatural amino acid mutagenesis methodology as the main theme.
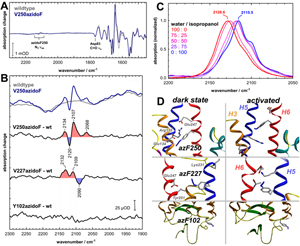
Research project I (FTIR difference spectroscopy elucidating activation mechanisms of rhodopsin) focuses on the fundamental understanding of activation mechanisms of rhodopsin, the visual pigment responsible for dim light vision and a prototypical member of the family A of GPCRs. FTIR is one of the most matured techniques to understand structural changes in rhodopsin compared to any other techniques such as X-ray crystallography, NMR, EPR, and fluorescent spectroscopy. It only requires microgram scale of purified protein, and it probes directly the global conformational changes of proteins. When proteins undergo conformational changes, FTIR difference spectra reveal distinct changes in the range from 1100cm-1 to 1800cm-1. Site-specific incorporation of p-azido-L-phenylalanine (Azp) into rhodopsin by the amber stop codon suppression technique provides a unique probe to study local conformational changes in proteins (Ye et al., Nature Chemical Biology, 2009). The anti-symmetric stretch of the azido probe absorbs in a spectral window around 2100 cm-1, which is free from other protein vibrations. This allows a precise identification and characterization of the vibrational band. In addition, the sensitivity of the azido stretch to the polarity and the electrostatic field offers an attractive solution to identify site-specific environmental changes during protein conformational transitions (Figure 2). We have incorporated Azp at selected sites in rhodopsin and monitor the vibration shift of the azido moiety during transitions between discrete conformational states including Lumi, Meta I and Meta II. This project involves the receptor expression in mammalian cells and receptor purification, as well as applications of FTIR spectroscopic technique. FTIR analyses have been accomplished in collaboration with Dr. Reiner Vogel at the Albert-Ludwigs-University Freiburg, Germany. Future direction is to adapt and translate this methodology to studies of other GPCR receptors such as beta2-adrenergic receptor.
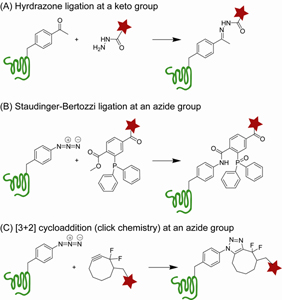 Research project II (Site-specific labeling of GPCR receptors for fluorescent imaging in live cells) aims to develop labeling method suitable for the fluorescent imaging detection of GPCRs in live cells. This imaging technique will allow one to directly probe the target GPCR. The advantage of this labeling method is the labeling of GPCR receptors can be site-specifically controlled through the genetic encoding of a reactive chemical handle. Ketones and azides epitomize the kinds of chemical handles that have been exploited for the purpose of site-specific fluorescent labeling in several model systems. Under physiological conditions, keto reacts specifically with hydrazide (or oxyamine) reagents through hydrazone (imine or oxime) ligation; while azide reacts specifically with phosphine through Staudinger ligation or with alkyne reagents through [3+2] cycloadditions (Figure 3). This project will involve incorporating p-acetyl-L-phenylalanine (Acp) or Azp at a solvent exposed site in a GPCR receptor through the amber stop codon suppression technology, and applying the hydrazone ligation or the Staudinger ligation to attach a fluorophore. It will also involve the establishment of a fluorophore library for the application of Staudinger reaction. Research project II (Site-specific labeling of GPCR receptors for fluorescent imaging in live cells) aims to develop labeling method suitable for the fluorescent imaging detection of GPCRs in live cells. This imaging technique will allow one to directly probe the target GPCR. The advantage of this labeling method is the labeling of GPCR receptors can be site-specifically controlled through the genetic encoding of a reactive chemical handle. Ketones and azides epitomize the kinds of chemical handles that have been exploited for the purpose of site-specific fluorescent labeling in several model systems. Under physiological conditions, keto reacts specifically with hydrazide (or oxyamine) reagents through hydrazone (imine or oxime) ligation; while azide reacts specifically with phosphine through Staudinger ligation or with alkyne reagents through [3+2] cycloadditions (Figure 3). This project will involve incorporating p-acetyl-L-phenylalanine (Acp) or Azp at a solvent exposed site in a GPCR receptor through the amber stop codon suppression technology, and applying the hydrazone ligation or the Staudinger ligation to attach a fluorophore. It will also involve the establishment of a fluorophore library for the application of Staudinger reaction.
|
|
Awards:
2005 - 2008
C. H. Li Memorial Scholar Award, Rockefeller University, New York, USA
1996
Challengers Cup Award for Achievement in Extracurricular Scientific Research in Organic Chemistry, Peking University, Beijing, China
1994 -1995
Excellent Student Award, Peking University, Beijing, China
|
|
Published Papers:
|
 |
7).
Ye, S., Huber, T., Vogel, R., & Sakmar, T.P.
FTIR analysis of GPCR activation using azido probes.
Nature Chemical Biology 5: 397-399 (2009).
PMID: 19396177 [PubMed - indexed for MEDLINE]
|
 |
6).
Ye, S.; Köhrer, C.; Huber, T.; Kazmi, M., Sachdev, P.; Yan, E.C.; Bhagat, A.; RajBhandary, U.L., & Sakmar, T.P.
Site-Specific Incorporation of Keto Amino Acids into Functional G Protein-Coupled Receptors Using Unnatural Amino Acid Mutagenesis.
J. Biol. Chem. 283: 1525-1533 (2008).
PMID: 17993461 [PubMed - indexed for MEDLINE]
|
|
5).
Ye, S., Discher, B.M., Strzalka, J., Xu, T., Wu S.P., Noy, D., Kuzmenko, I., Gog, T., Therien, M.J., Dutton, P.L. & Blasie, J.K.
Amphiphilic Four-Helix Bundle Peptides Designed for Light-Induced Electron Transfer Across a Soft Interface.
Nano Letters. 5:1658-1667 (2005).
PMID: 16159202 [PubMed - indexed for MEDLINE]
|
|
4).
Discher, B.M., Noy, D., Strzalka, J., Ye, S., Moser, C.C., Lear, J.D., Blasie, J.K., & Dutton, P.L.
Design of Amphiphilic Protein Maquettes: Controlling Assembly, Membrane Insertion, and Cofactor Interactions.
Biochemistry 44:12329-12343 (2005).
PMID: 16156646 [PubMed - indexed for MEDLINE]
|
|
3).
Ye, S., Churbanova I.Y., Strzalka J., Johansson J.S., & Blasie J.K.
A Designed Integral Membrane Protein Probes the Structural Features of Volatile Anesthetic Binding.
Biophysical Journal, 87, 4065-4074 (2004).
[not indexed on PUBMED]
|
|
2).
Ye, S., Strzalka J., Discher B.M., Dror N., Zheng S., Dutton P.L., & Blasie J.K.
Amphiphilic 4-Helix Bundles Designed for Biomolecular Materials Applications.
Langmuir, 20, 5897-5904 (2004).
PMID: 16459607 [PubMed - indexed for MEDLINE]
|
|
1).
Ye, S., Strzalka J., Chen X., Moser C.C., Dutton P.L., Ocko B.M., & Blasie J.K.
Assembly of a Vectorially-Oriented Four-Helix Bundle at the Air/Water Interface via Directed Electrostatic Interactions
Langmuir, 19, 1515-21 (2003).
[not indexed on PUBMED]
|
|
Recent Presentations:
|
|
1)
Ye, S.; Huber, T., Sakmar, T.P.
Probing conformational changes in rhdopsin with site-specific azido labels.
Biophysical Society Meeting, Boston, US, March, 2009. |
|
2)
Ye, S.; Huber, T., Sakmar, T.P.
Fluorescent Labeling of GPCRs by the Site Specific Incorporation of Unnatural Amino Acids.
Rockefeller University Postdoctoral Retreat, New York, September 2006.
^
|
|
Posters: |
|
8).
Ye, S.; Huber, T., Sakmar, T.P.
Fluorescent Labeling of GPCRs by the Site Specific Incorporation of Keto and Azido Amino Acids.
Biophysical Society Meeting, Boston, US, March, 2009.
|
|
7).
Ye, S.; Huber, T., Sakmar, T.P.
Site-Specific Labeling of Heptahelical Receptors Containing Keto and Azido Amino Acids.
Keystone symposia, Cambridge, UK, September, 2008.
|
|
6).
Ye, S.; Huber, T., Sakmar, T.P.
Fluorescent Labeling of GPCRs by the Site Specific Incorporation of Unnatural Amino Acids.
Rockefeller University Postdoctoral Retreat, New York, September 2007 and 2008.
|
|
5).
Ye, S.; Strzalka, J.W., Discher, B.M.; Noy, D., Moser, C.C.; Dutton, P.L., Blasie, J.K.
Design and characterization of artificial integral membrane proteins for vectorial electron transfer across soft interfaces.
226th ACS National Meeting, New York, September, 2004.
|
|
4).
Ye, S.; Strzalka, J.W., Churbanova, I.Y., Johansson, J.S., Blasie, J.K.
Model Membrane Protein for Binding Volatile Anesthetics.
48th Biophysical Society Annual Meeting, Baltimore, February, 2004.
|
|
3).
Ye, S.; Discher, B.M., Strzalka, J.W., Moser, C.C., Dutton, P.L., Blasie, J.K.
Design and physical characterization of vectorially oriented minimal electron-transfer protein monolayer at the air/water interface.
ACS National Meeting, New York, September, 2003.
|
|
2).
Ye, S.; Discher, B.M., Strzalka, J.W., Moser, C.C., Dutton, P.L., Ocko, B.M., Blasie, J.K.
Design and Physical Characterization of Vectorially Oriented Heme-Binding Peptide Monolayer.
36th Middle Atlantic Regional Meeting of ACS, Princeton, June, 2003.
|
|
1).
Ye, S.; Discher, B.M., Chen, X.X., Moser, C.C., Dutton, P.L., Ocko, B.M., Blasie, J.K.
Maquette Peptide Design and Its Applications in Biomaterial Engineering.
Institute of Medicine and Engineering of the University of Pennsylvania Annual Meeting, Philadelphia, Pennsylvania, December, 2001.
|

 Shixin Ye, Postdoctoral Associate
Shixin Ye, Postdoctoral Associate
